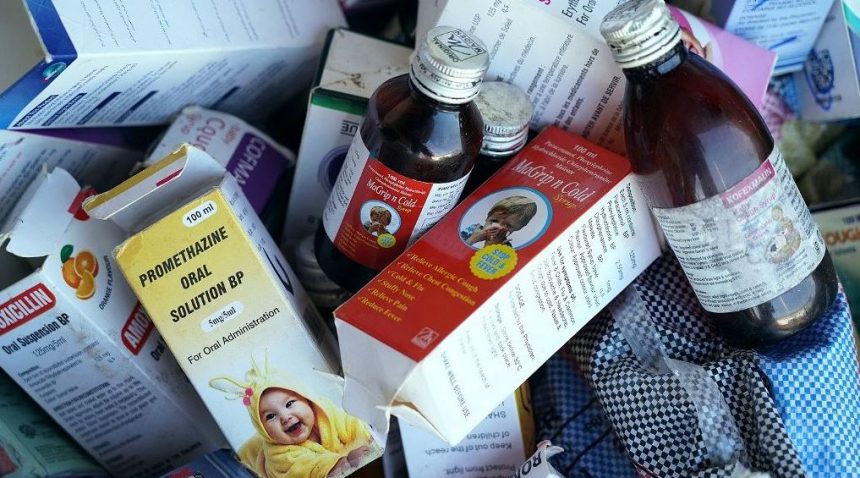
After at least 70 children passed away from acute kidney failure, a Gambian parliamentary committee recommended in a report made public on Tuesday that the company’s production facility in northern India be investigated and its products be prohibited in The Gambia.
Read also: Grief, fear, and rage over deadly cough syrup in the Gambia
On October 26, a commission was created to look into the causes of the fatalities.
In a report released on Tuesday, the commission recommended that “the government pursue legal action against Maiden Pharmaceuticals for shipping tainted medications to The Gambia.”
a commission “suggests placing all Maiden Pharmaceuticals goods on a blacklist and preventing them from being sold in the Gambian market. Its members claim to be “convinced that Maiden Pharmaceuticals exported the contaminated medications linked to at least 70 child deaths in The Gambia in 2022 and that they should be held accountable.
In the wake of these kids’ deaths, The Gambia recalled a number of medications in October.
The government announced the removal of all cough and cold medications from the market as well as all Maiden Pharmaceuticals-produced goods.
The World Health Organization (WHO) has launched investigations to ascertain whether the administration of these medications, which the UN agency claimed contained “unacceptable” amounts of diethylene glycol and ethylene glycol, which are frequently used as antifreeze and which can be fatal if ingested, caused the premature death of the 70 children.
Read also: WHO Warns That Cough Syrup May Be Found in Other Countries After 66 Deaths in The Gambia
The Maiden Pharmaceuticals production facility was shut down, Indian officials stated in October.